
The pharmaceutical industry ranks among the most innovative and highest-grossing sectors globally. In 2024, the European pharmaceutical market generated sales of around 280 billion US dollars. In Germany alone, sales in the first quarter of 2025 reached approximately 16.6 billion euros, positioning the country as the largest pharmaceutical market in Europe and the fourth largest worldwide. In parallel, packaging technology has grown in strategic importance. Beyond its core role in the safe storage and transport of medicines, it is increasingly a focal point for sustainability, regulatory compliance and the digital transformation of production and logistics.
Regulation remains a central driver. The Packaging & Packaging Waste Regulation (PPWR) will also affect the pharmaceutical and medical technology sector over the long term. Although the regulation does not initially apply to the packaging of medical products and pharmaceuticals, with transitional periods running until 2035 and 2040 respectively, industry action is already required. The pace of development is set to be visible at interpack 2026, from recyclable blister materials to digitalised production lines that document and optimise processes in real time.
Sustainability is drawing more attention across primary and secondary packaging. Some options, such as reusable or refillable formats, are difficult to implement for pharmaceuticals, and the use of recyclates is tightly limited by regulation. Nevertheless, for primary packaging, manufacturers have introduced alternatives to standard PVC and aluminium blister packs. Initial market solutions include moulded and lidding films made of PET or PP, as well as paper-based structures with barrier properties. In one joint project, four packaging companies developed a polypropylene pharmaceutical blister that meets stringent sector requirements. PP blister film from Etimex was tested and optimised for practical use on a modern blister machine from interpack exhibitor Uhlmann Pac-Systeme, under realistic production conditions.
Recyclable materials for pharmaceutical products
Material changes are being matched by advances in processing. Many machine builders are adapting equipment to run recyclable monomaterials while maintaining output and quality. Romaco, for example, produces blister packs made of recyclable PET monomaterial on the new Noack N 760 plate sealing machine. In four-lane operation, it achieves up to 150 PET/PET blisters per minute. The universally applicable intermittent blister platform covers a broad application spectrum, from solid dosage forms and ampoules to medical products and semi-solid forms.
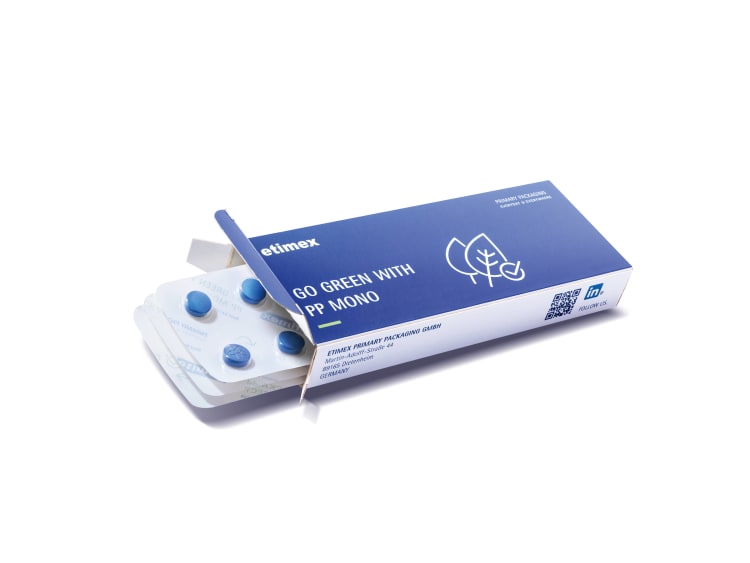
Four packaging companies, including Uhlmann Pac-Systeme, have jointly developed sustainable pharmaceutical blister packaging made from monomaterial. (Photo: Etimex)
Digitalisation and robotics in the pharmaceutical process
Recent machine developments are characterised by modularity, higher levels of automation and the use of robotics. Many of these technologies were first shown to a broad professional audience at interpack and have since shaped production standards.
Steriline combines robotics with a 3D vision system, referred to as 3D CPS, to support safe primary packaging of sensitive medicines. The system reduces particle emissions and enables flexible, low-contamination workflows. It recognises objects in the working area and adapts robot movements in real time, which allows conventional feeding systems such as hoppers or vibrating bowls to be replaced. The 3D CPS was developed together with ISS - Innovative Security Solutions, a spin-off of the Politecnico di Milano, aligning pharmaceutical process expertise with advanced robotics and image processing.

Steriline combines robotics with a 3D vision system. (Photo: Steriline)
Christ Packing Systems has secured a patent for a sealing unit tailored to pharmaceutical and medical technology requirements. Unlike conventional machines that seal from below, the FilmTeq 250 seals from above. According to the manufacturer, the sealing table remains unheated, which limits product temperature rise and reduces cooling demand. The top-down process also enables a more compact machine layout with a smaller footprint.

The patented FilmTeq sealing system seals gently from above. (Photo: Christ Packing System)
Mechanical engineering specialists are also targeting aseptic performance. In 2024, Syntegon reported record results driven by rising demand for complete aseptic solutions. The company introduced a new line concept for liquid pharmaceuticals, developed with two pharmaceutical partners. The SynTiso concept supports faster aseptic transportation and up to 50 percent shorter batch changes in a smaller space. With 100 percent in-process control, up to 600 syringes, vials or cartridges can be processed per minute, a level highlighted by the manufacturer as unprecedented. The system also addresses vaccine production needs and is suitable for filling ready-to-use containers.

SynTiso enables rapid aseptic transportation. (Photo: Syntegon)
Smart packaging: from NFC to functional labels
Beyond the ecological dimension, digital and functional features are becoming integral to pharmaceutical packaging. RFID chips, NFC labels and battery-free Bluetooth sensors provide real-time information on location, temperature and package condition. Smart blisters can additionally register removal events for individual tablets, enabling documentation of dosing times. Paper-based VOID security labels from Securikett offer tamper evidence and can be recycled together with the folding box they seal. Electronic package leaflets reduce paper use and improve access to up-to-date information for patients and healthcare professionals.

Paper-based VOID labels offer tamper protection and are recyclable. (Photo: Securikett)
Interpack 2026 will again showcase tailored solutions and new concepts along the entire pharmaceutical packaging value chain. Companies that invest in research, digital infrastructure and recyclable materials today are likely to secure long-term advantages. The focus will increasingly shift from the active ingredient to how safely, sustainably and intelligently a medicine reaches the patient.



