Kraiburg TPE, meanwhile, recently launched a new line of thermoplastic elastomers that not only hold certifications for healthcare applications in accordance with European Union and U.S. Food and Drug Administration standards but can also be combined directly with polyamides. Dubbed the MC/AD/PA Thermolast M series, Kraiburg says it is now introducing “the world’s first TPEs for medical applications in composites with polyamides – including transparent PA12.” The compounds are fully certified and suitable for a variety of attractive medical devices, including those used for in vitro diagnostics.

Kraiburg’s new TPE can combine with nylon in medical devices
PolyOne Corp. also supplies a number of materials for use in healthcare applications, including for catheters and tubing, and various medical devices. Recently, though, it tackled a slightly different challenge – helping a nonprofit charity called Global Vision 2020 to create a simple, effective way to bring clear eyesight to people living in extreme poverty. The resulting diagnostic device, called USee™, allows minimally trained practitioners in the field to accurately test the eyesight of people in impoverished areas. PolyOne’s IQ Design unit helped design the device and provided the medical-grade polycarbonate for the rectangular lenses used to help diagnose the patient’s prescription vision needs.
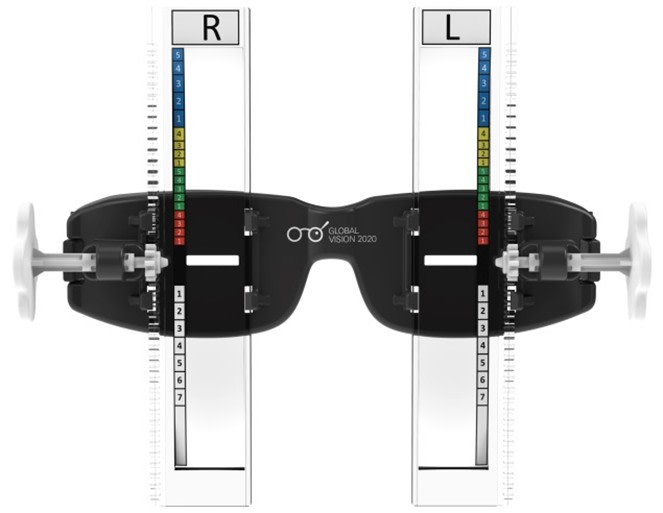
PolyOne partners with nonprofit to bring better vision to the masses
Another materials firm, Germany’s Covestro, worked closely with Ohio-based medical equipment maker Enable Injections to create a new, on-body drug-delivery system to help patients who need biologic drugs derived from organic sources to treat cancer, diabetes and other diseases.

Covestro materials help Enable on-body drug delivery
Biologics need to be injected or infused. Typically, this has required inconvenient visits to specialty healthcare facilities or painful self-injections of high-viscosity medications. Now, patients can wear this Enable device and easily self-administer the doses they need, when they need them. The new system – made with Covestro’s Makrolon® Rx1805 polycarbonate in a purple tint, and its Bayblend® M850 XF PC/ABS blend – provides the necessary safety, durability and bio-compatibility while being aesthetically pleasing.
And nowhere is plastics more prevalent in healthcare than in packaging. Most drugs are dispensed in some sort of plastic bottle, container or foil-backed blister pack, and packagers increasingly are adding “smart” technologies to such products to improve safety while also helping users to keep track of the medications they are taking.
Austrian packaging group Alpla, for example, recently introduced CRC justONE, a very light, childproof closure, manufactured in just a single injection molding process with straightforward assembly. Normally, there are three parts to a childproof closure with a tamper-evident band that can only be opened by simultaneously pushing and turning the closure. And these parts typically are produced in three separate production steps and assembled later. This new production process from ALPLApharma, Alpla’s newly consolidated healthcare brand, has now streamlined this into a significantly more efficient workflow.

Alpla’s new child-proof closure simplifies production
National Geographic asked the right question recently. And, in short, the current answer is, “No, safe, efficient medical care today cannot exist without plastic.”
Come to Chinaplas 2020 in Shanghai this April 21-24 to see first-hand some of the amazing technologies that are helping to enable modern medicine. For more information about Chinaplas 2020, please visit the official show website at www.ChinaplasOnline.com.



